



In 1900, Austrian Biologist Karl Landsteiner discovered the existence of blood groups, leading to the developments in transfusion medicine. Since then, transfusion medicine has developed into one of the most basic medical treatment modalities. While methods for blood preservation are advancing, transfusion is still donor-dependent and relies on donation.
Now, more than a 100 years later, research teams in The University of Tokyo and Kyoto University have succeeded in developing techniques to produce platelets from induced pluripotent (iPS) cells. These techniques have enabled the stable production of safe and low-cost platelets without relying on blood donations. This technological innovation can be considered as a long-awaited second revolution in transfusion medicine.
iPS cells
Megakaryon Corporation was founded to employ the application of iPS cells in platelet production, thereby establishing techniques to manufacture safe and low-cost platelets which can be produced on demand. With cooperation from both domestic and foreign academic institutions and companies, Megakaryon is hoping to achieve the second revolution in transfusion medicine and contribute to the global evolution of medical treatment.

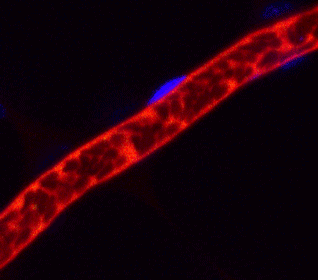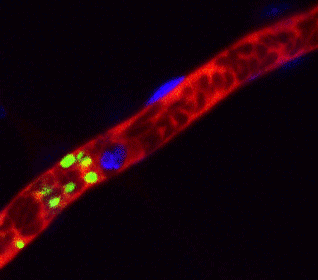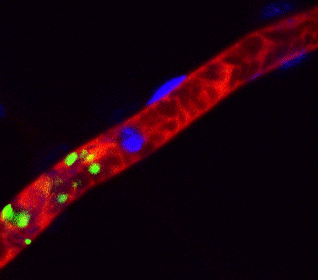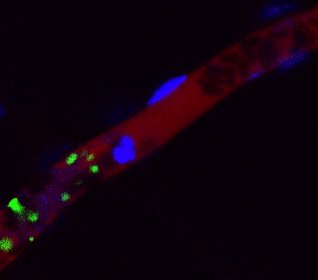
Arrest of bleeding in the blood vessel (red) of a mouse with platelet (green) produced from human iPS cells
Platelets, also known as thrombocytes, are major blood components that play a crucial role in hemostasis. On endothelial damage, platelets get activated leading to the adhesion and aggregation at the wound site, there by stopping bleeding. Platelets are required in large quantities during surgeries and are indispensable in medical establishments. While other blood components can be refrigerated and stored for longer periods, platelets can be stored only at room temperature under constant agitation and is viable for only four days.

Like the different red blood cell antigens (A, B, AB and O), there are different human leukocyte antigens (HLAs) present on the white blood cells. Patients who undergo repeated blood transfusions can develop HLA cross-reactivity when their body produces antibodies against the foreign HLAs introduced through transfusion. Due to this reason, HLA-matched platelets are recommended for such patients. However, the probability of finding a potential HLA-matched donor is very rare: one in four among siblings, and one in tens of thousands among unrelated individuals.
For regenerative therapy using platelet products, a huge number of cells are required to achieve the final product. For example, only 10,000 retinal pigment cells, 1,000,000 dopamine regenerative cells or 10,000,000 neural stem cells are required per respective treatment, while 200-300 billion cells are required to prepare enough platelets for a single dose of platelet transfusion. For repeated transfusions, each dose is prepared separately which requires 200-300 billion cells every time. Considering these factors, medical experts expect Megakaryon’s research and development (R&D) team to develop a HLA-matched platelet mass-manufacturing technology.
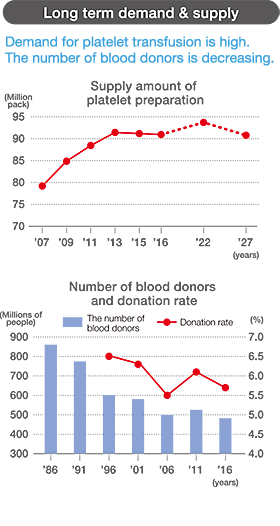
Transfusion:86% from elder than or equal to 50 years old
Donation:76% from less than 50 years old
Supply results until FY 2016. Demand forecast after 2017
